New Tag Alternatives Pulsatile or Continuous Flow
Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: is there an advantage to pulsatility?
Introduction
Left ventricular assist devices (LVADs) have gained widespread use as bridge-to-transplant (BTT) and destination therapy for advanced congestive heart failure. Currently, the most commonly used devices are continuous-flow left ventricular assist devices (CFVADs), including axial-flow and centrifugal-flow pumps. Compared to the first generation pulsatile-flow VADs (PFVADs), CFVADs are smaller, more reliable, and more durable. However, despite their increasing use, CFVADs have been associated with complications including gastrointestinal bleeding, arteriovenous malformations, hemolysis, pump thrombosis and aortic insufficiency (AI). There is speculation that the decrease in arterial pulsatility with CFVAD support could be contributing to these complications. CFVADs produce a new hemodynamic milieu that is characterized by continuous-flow (CF) and diminished pulsatility systemically. The rate of left ventricle recovery seems to be lower with CFVADs compared to PFVADs. Based on these observations, there is currently an interest in developing control algorithms for CFVADs as a method to generate a pulse pressure (PP), in an attempt to decrease the CFVAD's associated adverse events and increase the rate of myocardial recovery by allowing physiologic loading/exercise and weaning.
Previous studies have compared CFVADs with PFVADs and have presented differing outcomes. It is important to understand and examine the outcome differences between CFVAD and PFVAD in order to further advance LVAD therapy. The objective of this review is to compare the outcomes of CFVADs and PFVADs, and examine the need for arterial pulsatility to minimize CFVAD associated complications and to improve the rate of myocardial recovery.
Pulsatility
The arterial pulse is an important part of our cardiovascular system. Human cells can detect and are adapted to the cyclic changes of pressure and flow. PP is quantified as the difference between maximal aortic systolic pressure and minimum aortic diastolic pressure. Pulsatility index (PI) is another common definition for quantifying pulsatility via flow. PI is the difference between peak systolic and minimum diastolic flow velocity, divided by the mean blood flow velocity. PI requires more advanced measuring instruments to measure than PP. Other definitions have been used and proposed to quantify the dynamic energy changes with blood flow, since pulsatility could be defined as a function of energy gradient, e.g., energy equivalent pressure (EEP) and surplus hemodynamic energy (SHE) (1).
Vascular responses to pulsatility
The physiologic and cellular responses to pulsatile blood flow have been studied extensively. Pulsatility creates shear and strain forces on the endothelium, smooth muscle and fibroblast cells in both the macro- and microcirculation. At the cellular level, the mechanical forces of pulsatility constantly induce various cellular signaling pathways and have significant effects on endothelium regulation of vasodilation and vascular remodeling, including matrix deposition, programed cell death, smooth muscle cell proliferation, and atherosclerosis. Multiple studies have that shown pulsatile flow (PF) has a greater impact on endothelial regulation than CF. Nakata et al. (2) showed that maximal flow rate with PF exerted a greater effect on wall shear stress than CF. Gambillara et al. (3) demonstrated that the reduction of pulsatility with reduced cyclic stretch exhibited a higher level of matrix degradation and affected vascular cell proliferation. Nishinaka et al. (4), in a study of prolonged continuous-flow left heart bypass, revealed that the aorta became significantly thinner (50% reduction) with an increased proportion of low contractility smooth muscle cells and reduced vascular sensitivity to phenylephrine. Hutcheson et al. (5) emphasized the importance of both the frequency and amplitude of PF in endothelium-derived vasodilation regulation. Nakano et al. (6) demonstrated that both the frequency and amplitude of PF are directly related to endothelial production of nitric oxide causing vasodilation. Thacher et al. (7) also demonstrated how reduced pulsatile pressure decreased bradykinin-dependent vascular relaxation, reduced nitric oxide production and increased vascular oxidative stress.
Furthermore, pulsatility plays a significant role in microcirculation and capillary beds. Orime et al. (8) compared the role of pneumatic PF pumps and centrifugal CF pumps in end-organ microcirculation in cardiogenic shock. They showed that the PF pump was more effective in improving and maintaining function and microcirculation of end organs when the liver tissue flow, renal cortex flow and stomach mucous flow were measured. Sezai and colleagues (9) also demonstrated that PF support provided better microcirculation in both the kidney and liver compared to CF support. While some have argued that the pulse is significantly lower and possibly undetectable at the capillary level, several group including Baba et al. (10) and Lee et al. (11) have shown that pulsatility is indeed present at the capillary level and that microcirculatory flow patterns are different between PF and CF support.
Outcomes comparison of CFVAD and PFVAD
Hemodynamic changes and LV unloading
In a mock circulatory loop and computer stimulation model, Koenig et al. (12) examined the differences in ventricular unloading between CFVADs and PFVADs, and showed that CFVAD support provided a larger reduction in left ventricular end-diastolic pressure and volume but also caused a disarrangement of the pressure-volume loop. Mean diastolic aortic pressure and mean arterial pressure were also higher with CFVAD. Bartoli et al. (13), continued the study with a chronic ischemic heart failure (HF) bovine model and compared PFVAD and CFVAD in vivo hemodynamic responses. CFVAD support provided greater LV unloading than PFVAD support; however, CFVAD also increased the LV systolic pressure and aortic mean aortic pressure, while pressure parameters with PFVAD were preserved. Cheng et al. (14) recently presented some of the results in humans comparing CFVAD (HeartMate II) and PFVAD (HeartMate XVE). Hemodynamic measurements and echocardiographic images were obtained at baseline (before LVAD implantation), intra-operatively, and post-operatively at 30, 90, 180 and 360 days. CFVAD and PFVAD had a similar reduction in LV end-diastolic pressure, external work and contractility, but aortic pressure and vascular resistance were higher with CFVAD (Table 1). On echocardiography, no significant difference in LV size, LV unloading or degree of mitral regurgitation was found between the two groups (Figure 1). Both CFVAD and PFVAD pressure unload the LV and reduce wall stress and external work, but CFVAD led to an increase in vascular resistance.
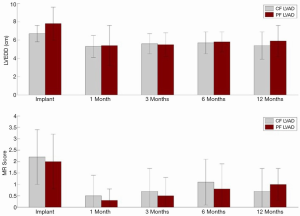
Figure 1 Echocardiographic findings of 36 patients (CFVAD n=17; PFVAD n=19). LVEDD, left ventricular end-diastolic dimension; MR, mitral regurgitation; CFVAD, continuous-flow left ventricular assist device; PFVAD, pulsatile-flow left ventricular assist device.
Garcia et al. (15) compared ventricular volume and pressure unloading in HeartMate XVE and HeartMate II (CFVAD n=20; PFVAD n=15) with a fixed pump speed and found that CFVAD and PFVAD unload LV equally, but PFVAD was able to produce a greater decrease in pulmonary pressure. Klotz et al. (16) examined LV pressure and volume unloading in 31 patients with CFVAD or PFVAD. LV pressure unloading and reduction of pulmonary pressure was similar in both groups, but LV volume unloading was more pronounced with PFVAD support. However, their PFVAD patients on average had larger preoperative LV volumes, which produced a greater overall reduction in volume postoperatively than the CFVAD group. Furthermore, the reported LVAD output flow in the PFVAD group was also significantly higher than that of the CFVAD group. Both differences between groups have made the results difficult to interpret and may suggest that the level of support could be just as important as device type in LV unloading. Thohan et al. (17) compared the Novacor (PFVAD) and DeBakey-Noon (CFVAD) in 20 patients and reported that PFVADs provided better ventricular unloading than CFVADs, in terms of left ventricular end diastolic and systolic volume and LV mass, but that both CFVADs and PFVADs equally reduced cellular markers of HF including tumor necrosis factor (TNF)-α. However, the level of support for either device type was not reported.
Letsou et al. (18), in a heart failure porcine model with equal level of device support, showed the mean aortic pressure and total cardiac output were higher and left atrial pressure was lower with PFVAD support compared to CFVAD support at the same flow rate, and hence provided superior left ventricular unloading. Gohean et al. (19) recently confirmed their findings using a novel computational cardiovascular system model.
LV remodeling and recovery
Multiple studies have shown that LVAD support can enable spontaneous myocardial recovery, which has led to successful LVAD removal in some heart failure patients (20-22). Farrar et al. (22) retrospectively evaluated 22 non-ischemic heart failure patients who were successfully weaned from LVAD (PFVAD) or biventricular assist devices after recovery of ventricular function, and compared them to 43 BTT patients who underwent transplantation. They reported that long-term survival for patients who recovered from LVAD support for acute cardiomyopathies and myocarditis was equivalent to that for cardiac transplantation. Krabatsch et al. (23) studied patients with dilated cardiomyopathy who underwent LVAD implantation (n=387), where 144 patients were implanted with PFVADs and 243 patients were implanted with CFVADs. Thirty-four patients recovered, and patients with PFVADs had three-fold higher chance of myocardial recovery than those with CFVADs. Kato et al. (24) studied remodeling in 61 patients (PFVAD n=31; CFVAD n=30) and concluded that PFVADs are more effective in inducing myocardial recovery, as indicated by their echocardiographic measurement of LV systolic and diastolic function and brain natriuretic peptide (BNP) and extracellular matrix (ECM) biomarkers measurements. Reduction in coronary flow could be one of the contributors to a lower rate of LV recovery in CFVAD. Ootaki et al. (25) and Giridharan et al. (26) reported significantly reduced coronary flow with increasing CFVAD support in animal models. Furthermore, the concern for cardiac atrophy has been reported with CFVAD support, in which it was correlated with the level and duration of CF support (27). Overall, from the studies above, PFVAD has a higher LV recovery rate with less physiologic derangement when compared to CFVAD at the same level of support.
Bridge-to-transplantation
Several studies have directly compared PFVAD and CFVAD outcomes in BTT patients. Ventura et al. (28) reported their study examining the differential impact of PFVADs (HeartMate XVE) and CFVADs (HeartMate II) on post-transplant outcomes utilizing the UNOS database from 2004-2009. With patient characteristics adjusted and paired, compared to PFVADs, patients with HMII (CFVADs) had similar one- and three-year survival after heart transplantation but with less risk of significant infection or early graft rejection. However, after three years post-transplant, HeartMate XVE patient survival dropped significantly while HeartMate II patient survival remained relatively stable. Klotz et al. (29) showed the post-transplant survival rate and BTT survival rate to be similar between CFVAD and PFVAD patients. However, the rate of rejection was lower with PFVAD (33% vs. 89%). Nativi et al. (30), examined the post-transplant survival in BTT patients supported with PFVADs or CFVADs, utilizing the International Society for Heart and Lung Registry during 2004-2008. They found no significant difference in post-transplant survival of patients supported with CFVADs or PFVADs.
End-organ perfusion
Some have proposed that PFVADs, with their higher pulsatility, provide better end-organ perfusion than CFVADs. Potapov et al. (31) examined the arterial wall histology of various end organs obtained from autopsy of CFVAD and PFVAD recipients (n=27). With a mean support of 467 days (PFVAD) and 263 days (CFVAD), no significant differences in the end organs arterial wall histology were found between the two groups. Saito et al. (32), in a sheep model, compared the effects of PF and CF on renal and hepatic function with support up to 340 days after LVAD placement. Histologic exams showed no changes in the end-organ function between groups. Radovancevic et al. (33) retrospectively compared end-organ function between CFVADs (Jarvik 2000 and HeartMate II) and PFVADs (HeartMate XVE) in humans, over a time span of 15 months. Markers of both hepatic and renal function were comparable between CFVAD and PFVAD. Kamdar et al. (34) reviewed hepatic and renal function in BTT patients within the first three months of LVAD implantation. The devices examined included an axial CFVAD, centrifugal CFVAD, and PFVAD. They found that all three devices provided equally adequate circulatory support to maintain end-organ function in patients with end-stage heart failure. Sakaki et al. (35) evaluated the pulmonary function with CF, and found no change in angiotensin-converting enzyme levels or extravascular water content with nonpulsatile support. Nishinaka et al. (36) and Wieselthaler et al. (37) examined cerebral metabolism and endocrine function with CF support and found no significant difference or impairment when compared to PF support. Most of the current aforementioned studies showed no significant differences in effect on end-organ function between CFVADs and PFVADs.
AI and fusion
AI has been reported with CFVAD support. Pak et al. reported a twofold increased incidence of AI in patients supported with CFVADs (HeartMate II) compared to those with PFVADs (HeartMate XVE) (38). A biomechanical mock loop study of aortic valve leaflets during CFVAD support by May-Newman et al. (39) found that the average aortic leaflet strain increased during CFVAD support and may lead to AI, with adversely effects on aortic root and leaflet remodeling. Hatano et al. (40) compared AI frequency with multiple CFVADs and PFVADs and showed CFVAD support to be an independent risk factor for the development of AI. AI was more common in patients when the aortic valve was chronically closed. Karmonik et al. (41) compared hemodynamic changes in the ascending aorta between CFVAD and PFVAD in humans. PFVAD was found to have a more favorable hemodynamic condition in the ascending aorta and a lower retrograde flow velocity toward the aortic valve when compared to CFVAD.
Aortic valve fusion is another complication seen with CFVAD support. Mudd et al. (42), in a retrospective evaluation of CFVAD BTT patients (HeartMate II), found that 88% of the patients supported with CFVADs had evidence of aortic valve commissural fusion. Aortic valve fusion has also been reported with PFVAD support. Rose et al. (43) reported thrombus organization at the aortic cusps causing aortic commissural fusion and chronic valve closure with PFVAD support. These data emphasized the importance of intermittent aortic valve opening to avoid valve strain and to reduce the risk of thrombus formation during either CFVAD or PFVAD support.
Gastrointestinal (GI) bleeding
Gastrointestinal bleeding has been reported starting with the earliest models of CFVAD support (Jarvik 2000) (44). John et al. (45) examined HeartMate II (CFVAD) and reported GI bleeding was observed in 14% of CFVAD patients. Crow et al. (46) compared bleeding events between CFVADs and PFVADs. CFVAD patients, who were smaller in body size and had a longer duration of CFVAD support, had a higher risk of GI bleeding. Furthermore, arteriovenous malformations were at least four times higher in the CFVAD group. In a study with HeartMate II patients (n=172), Demirozu et al. (47) reported 19% of the HeartMate II patients have GI bleeding and 30% of these patients had associated AVM. In a retrospective review, Stern et al. (48) reported patients with a HeartMate II (CFVAD) had a higher GI bleeding rate than other devices. Forty percent of the HeartMate II recipient experienced at least one episode of GI bleeding, which was related to patient age and preoperative use of aspirin. Acquired Von Willebrand Syndrome has also been seen with CFVAD (HeartMate II) support and may be associated with the higher prevalence of GI bleeding (49). CFVADs can cause damage to the Von Willebrand factor (VMF) multimers in a similar mechanism to aortic stenosis. However, while decreased VMF occurred in almost all HeartMate II patients, GI bleeding occurred in a smaller proportion. Overall, the combination of increased AVM formation and reduced VMF with CFVAD support may contribute to the higher incidence of GI bleeding seen with nonpulsatile support.
Right heart failure
CFVADs can unload the LV rapidly, but this may also contribute to altered right ventricular (RV) geometry and septal position, and adversely affect RV function. Patel et al. (50) compared HeartMate II (CFVAD) and HeartMate I (PFVAD) and demonstrated similar frequency of right HF with either device support. However, fewer CFVAD patients required right ventricular assist device (RVAD) support. Kormos et al. (51) reported only that 6% of patients with CFVADs (HeartMate II) support required RVAD support in comparison to >37% in patients with PFVADs. Takeda et al. (52) studied patients who required RVAD insertion after LVAD (CFVAD or PFVAD) placement (n=282). While the timing of RVAD was irrelevant to the outcomes, the one year survival rate was 82% for the non-RVAD group and 40% for the RVAD group. The incidence of severe RV failure requiring RVAD support was significantly higher with PFVADs (20%) compared to CFVADs (5.3%). Ozturk et al. (53) compared CFVADs (HeartWare) and PFVADs (EXCOR) in terms of pulmonary artery pressure and right heart function in 27 patients. Both were found to be equally effective in reducing mean pulmonary artery pressure, while a significantly greater decrease in systolic pulmonary pressure was noticed in patients with CFVADs. Slaughter et al. (54), in a randomized study, showed that 20% of CFVAD patients had right heart failure requiring extended use of inotropes in comparison to 27% of PFVAD patients. However, the incidence of right heart failure requiring RV mechanical support was similar between the two groups.
Discussion
Pulsatility is an innate part of our cardiovascular physiology and function. With the advancement in device technology, improvements in size, durability, reliability, and efficacy of the current generation CFVADs have certainly shifted the clinical focus from the first generation PFVADs to CFVADs in treating advanced heart failure. But with studies showing CFVAD may have its own specific associated complications and a lower rate of LV recovery, PFVAD support or a pulsatility control algorithm in CFVAD could prove beneficial and potentially necessary for long term support. Flow modulation control strategies are currently being examined to generate pulsatility in centrifugal CFVADs. The Heartware HVAD (Heartware, Inc., Framingham, MA) uses pump speed modulation by modulating speed through a Lavare cycle, allowing intermittent aortic valve opening for washing of the aortic root (55). Although the HVAD speed modulating function is intended to avoid thrombus formation, this capability is currently being further developed to induced greater pulsatility (56,57). The new HeartMate III (Thoratec, Corp, Pleasanton, CA) includes a pulse mode (58,59) which can produce near-physiologic PP, as demonstrated in an animal model and mock circulation loops. However, the current tested HeartMate III models can only generate PP of about 25 mmHg, although further studies are in progress. Various methods attempting to increase the ability of centrifugal CFVADs to produce PF have been proposed and are currently being developed, including the use of a trapezoidal profile and sinusoidal and synchronous flow modulation strategy with an adaptive physiological controller (60-62).
It is still unknown how much pulsatility is sufficient in order to normalize vascular responses, avoid specific CFVAD-related complications and improve the rate of myocardial recovery. Several obstacles to research include the lack of a universal metric in quantifying pulsatility and definition of CFVAD-generated pulse vs. physiological pulse (pulsatility types with different energy patterns). Future studies focusing on examining the physiologic and hemodynamic responses, end-organ function, LV remodeling (both clinical and cellular level), with varying degrees of pulsatility, device support levels and duration are needed. Furthermore, the myocardial recovery rate, allowing for LVAD explantation, still remains low for both PFVAD and CFVAD, despite their differences. Additional interventions, as a supplement to LVAD support, such as a specific drug regimen, stem cell therapy and ECM injection may also be needed to facilitate recovery and avoid heart transplantation.
In conclusion, CFVADs have contributed significantly to the growth and success of mechanical circulatory support for advanced heart failure as either BTT or DT. CFVADs are significantly smaller with increased reliability and durability which has resulted in improved survival. Over several years of support, despite diminished pulsatility, routine lab tests would suggest adequate end organ function. However, with more patients receiving devices for longer periods of time, it appears as though there may be adverse events that are associated with the diminished pulsatility, increased pressure gradients on the aortic valve and decreased compliance in smaller arterial vessels. There are ongoing efforts to try to create a pulse while preserving the advantages of the smaller CFVADs. It appears as though the ultimate solution will be a return to re-creating our natural physiology.
Acknowledgements
Disclosure: Slaughter: HeartWare, Inc-research grant support.
References
- Soucy KG, Koenig SC, Giridharan GA, et al. Defining pulsatility during continuous-flow ventricular assist device support. J Heart Lung Transplant 2013;32:581-7. [PubMed]
- Nakata M, Tatsumi E, Tsukiya T, et al. Augmentative effect of pulsatility on the wall shear stress in tube flow. Artif Organs 1999;23:727-31. [PubMed]
- Gambillara V, Thacher T, Silacci P, et al. Effects of reduced cyclic stretch on vascular smooth muscle cell function of pig carotids perfused ex vivo. Am J Hypertens 2008;21:425-31. [PubMed]
- Nishinaka T, Tatsumi E, Nishimura T, et al. Change in vasoconstrictive function during prolonged nonpulsatile left heart bypass. Artif Organs 2001;25:371-5. [PubMed]
- Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol 1991;261:H257-62. [PubMed]
- Nakano T, Tominaga R, Morita S, et al. Impacts of pulsatile systemic circulation on endothelium-derived nitric oxide release in anesthetized dogs. Ann Thorac Surg 2001;72:156-62. [PubMed]
- Thacher T, Gambillara V, da Silva RF, et al. Reduced cyclic stretch, endothelial dysfunction, and oxidative stress: an ex vivo model. Cardiovasc Pathol 2010;19:e91-8. [PubMed]
- Orime Y, Shiono M, Nakata K, et al. The role of pulsatility in end-organ microcirculation after cardiogenic shock. ASAIO J 1996;42:M724-9. [PubMed]
- Sezai A, Shiono M, Orime Y, et al. Major organ function under mechanical support: comparative studies of pulsatile and nonpulsatile circulation. Artif Organs 1999;23:280-5. [PubMed]
- Baba A, Dobsak P, Saito I, et al. Microcirculation of the bulbar conjunctiva in the goat implanted with a total artificial heart: effects of pulsatile and nonpulsatile flow. ASAIO J 2004;50:321-7. [PubMed]
- Lee JJ, Tyml K, Menkis AH, et al. Evaluation of pulsatile and nonpulsatile flow in capillaries of goat skeletal muscle using intravital microscopy. Microvasc Res 1994;48:316-27. [PubMed]
- Koenig SC, Pantalos GM, Gillars KJ, et al. Hemodynamic and pressure-volume responses to continuous and pulsatile ventricular assist in an adult mock circulation. ASAIO J 2004;50:15-24. [PubMed]
- Bartoli CR, Giridharan GA, Litwak KN, et al. Hemodynamic responses to continuous versus pulsatile mechanical unloading of the failing left ventricle. ASAIO J 2010;56:410-6. [PubMed]
- Cheng A, Soucy KG, Giridharan GA, et al. Left Ventricular and Vascular Biomechanics in Advanced Heart Failure Patients Supported by Continuous and Pulsatile LVADS. Presented at 2013 Annual Academic Surgical Congress, San Diego, CA, 2013.
- Garcia S, Kandar F, Boyle A, et al. Effects of pulsatile- and continuous-flow left ventricular assist devices on left ventricular unloading. J Heart Lung Transplant 2008;27:261-7. [PubMed]
- Klotz S, Deng MC, Stypmann J, et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg 2004;77:143-9; discussion 149-50. [PubMed]
- Thohan V, Stetson SJ, Nagueh SF, et al. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: a comparative analysis with pulsatile-type devices. J Heart Lung Transplant 2005;24:566-75. [PubMed]
- Letsou GV, Pate TD, Gohean JR, et al. Improved left ventricular unloading and circulatory support with synchronized pulsatile left ventricular assistance compared with continuous-flow left ventricular assistance in an acute porcine left ventricular failure model. J Thorac Cardiovasc Surg 2010;140:1181-8. [PubMed]
- Gohean JR, George MJ, Pate TD, et al. Verification of a computational cardiovascular system model comparing the hemodynamics of a continuous flow to a synchronous valveless pulsatile flow left ventricular assist device. ASAIO J 2013;59:107-16. [PubMed]
- Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873-84. [PubMed]
- Dandel M, Weng Y, Siniawski H, et al. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 2005;112:I37-45. [PubMed]
- Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant 2002;21:516-521. [PubMed]
- Krabatsch T, Schweiger M, Dandel M, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg 2011;91:1335-40. [PubMed]
- Kato TS, Chokshi A, Singh P, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail 2011;4:546-53. [PubMed]
- Ootaki Y, Kamohara K, Akiyama M, et al. Phasic coronary blood flow pattern during a continuous flow left ventricular assist support. Eur J Cardiothorac Surg 2005;28:711-6. [PubMed]
- Giridharan GA, Ewert DL, Pantalos GM, et al. Left ventricular and myocardial perfusion responses to volume unloading and afterload reduction in a computer simulation. ASAIO J 2004;50:512-8. [PubMed]
- Kinoshita M, Takano H, Taenaka Y, et al. Cardiac disuse atrophy during LVAD pumping. ASAIO Trans 1988;34:208-12. [PubMed]
- Ventura PA, Alharethi R, Budge D, et al. Differential impact on post-transplant outcomes between pulsatile- and continuous-flow left ventricular assist devices. Clin Transplant 2011;25:E390-5. [PubMed]
- Klotz S, Stypmann J, Welp H, et al. Does continuous flow left ventricular assist device technology have a positive impact on outcome pretransplant and posttransplant? Ann Thorac Surg 2006;82:1774-8. [PubMed]
- Nativi JN, Drakos SG, Kucheryavaya AY, et al. Changing outcomes in patients bridged to heart transplantation with continuous- versus pulsatile-flow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2011;30:854-61. [PubMed]
- Potapov EV, Dranishnikov N, Morawietz L, et al. Arterial wall histology in chronic pulsatile-flow and continuous-flow device circulatory support. J Heart Lung Transplant 2012;31:1171-6. [PubMed]
- Saito S, Westaby S, Piggot D, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg 2002;74:1080-5. [PubMed]
- Radovancevic B, Vrtovec B, de Kort E, et al. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant 2007;26:815-8. [PubMed]
- Kamdar F, Boyle A, Liao K, et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant 2009;28:352-9. [PubMed]
- Sakaki M, Taenaka Y, Tatsumi E, et al. Influences of nonpulsatile pulmonary flow on pulmonary function. Evaluation in a chronic animal model. J Thorac Cardiovasc Surg 1994;108:495-502. [PubMed]
- Nishinaka T, Tatsumi E, Nishimura T, et al. Effects of reduced pulse pressure to the cerebral metabolism during prolonged nonpulsatile left heart bypass. Artif Organs 2000;24:676-9. [PubMed]
- Wieselthaler GM, Riedl M, Schima H, et al. Endocrine function is not impaired in patients with a continuous MicroMed-DeBakey axial flow pump. J Thorac Cardiovasc Surg 2007;133:2-6. [PubMed]
- Pak SW, Uriel N, Takayama H, et al. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant 2010;29:1172-6. [PubMed]
- May-Newman K, Enriquez-Almaguer L, Posuwattanakul P, et al. Biomechanics of the aortic valve in the continuous flow VAD-assisted heart. ASAIO J 2010;56:301-8. [PubMed]
- Hatano M, Kinugawa K, Shiga T, et al. Less frequent opening of the aortic valve and a continuous flow pump are risk factors for postoperative onset of aortic insufficiency in patients with a left ventricular assist device. Circ J 2011;75:1147-55. [PubMed]
- Karmonik C, Partovi S, Schmack B, et al. Comparison of hemodynamics in the ascending aorta between pulsatile and continuous flow left ventricular assist devices using computational fluid dynamics based on computed tomography images. Artif Organs 2014;38:142-8. [PubMed]
- Mudd JO, Cuda JD, Halushka M, et al. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant 2008;27:1269-74. [PubMed]
- Rose AG, Park SJ, Bank AJ, et al. Partial aortic valve fusion induced by left ventricular assist device. Ann Thorac Surg 2000;70:1270-4. [PubMed]
- Letsou GV, Shah N, Gregoric ID, et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J Heart Lung Transplant 2005;24:105-9. [PubMed]
- John R. Current axial-flow devices--the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg 2008;20:264-72. [PubMed]
- Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009;137:208-15. [PubMed]
- Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2011;30:849-53. [PubMed]
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg 2010;25:352-6. [PubMed]
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207-13. [PubMed]
- Patel ND, Weiss ES, Schaffer J, et al. Right heart dysfunction after left ventricular assist device implantation: a comparison of the pulsatile HeartMate I and axial-flow HeartMate II devices. Ann Thorac Surg 2008;86:832-40; discussion 832-40. [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [PubMed]
- Takeda K, Naka Y, Yang JA, et al. Timing of temporary right ventricular assist device insertion for severe right heart failure after left ventricular assist device implantation. ASAIO J 2013;59:564-9. [PubMed]
- Ozturk P, Engin AY, Nalbantgil S, et al. Comparison of continuous-flow and pulsatile-flow blood pumps on reducing pulmonary artery pressure in patients with fixed pulmonary hypertension. Artif Organs 2013;37:763-7. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Larose JA, Tamez D, Ashenuga M, et al. Design concepts and principle of operation of the HeartWare ventricular assist system. ASAIO J 2010;56:285-9. [PubMed]
- Koenig SC, Slaughter MS, et al. ASAIO 58th Annual Conference. San Francisco, CA. 2012.
- Ising MS, Sobieski MA, Slaughter MS, et al. Hemodyanmic responses to continuous flow LVAD speed modulation in computer simulation and in-vitro mock loop studies. 19th Congress of the International Society of Rotary Blood Pumps. Louisville, KY, 2011.
- Farrar DJ, Bourque K, Dague CP, et al. Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J 2007;53:310-5. [PubMed]
- Bourque K, Dague C, Farrar D, et al. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artif Organs 2006;30:638-42. [PubMed]
- Bearnson GB, Olsen DB, Khanwilkar PS, et al. Pulsatile operation of a centrifugal ventricular assist device with magnetic bearings. ASAIO J 1996;42:M620-4. [PubMed]
- Cox LG, Loerakker S, Rutten MC, et al. A mathematical model to evaluate control strategies for mechanical circulatory support. Artif Organs 2009;33:593-603. [PubMed]
- Vandenberghe S, Segers P, Antaki JF, et al. Hemodynamic modes of ventricular assist with a rotary blood pump: continuous, pulsatile, and failure. ASAIO J 2005;51:711-8. [PubMed]
Cite this article as: Cheng A, Williamitis CA, Slaughter MS. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: is there an advantage to pulsatility? Ann Cardiothorac Surg 2014;3(6):573-581. doi: 10.3978/j.issn.2225-319X.2014.08.24
Source: https://www.annalscts.com/article/view/5143/html
0 Response to "New Tag Alternatives Pulsatile or Continuous Flow"
Post a Comment